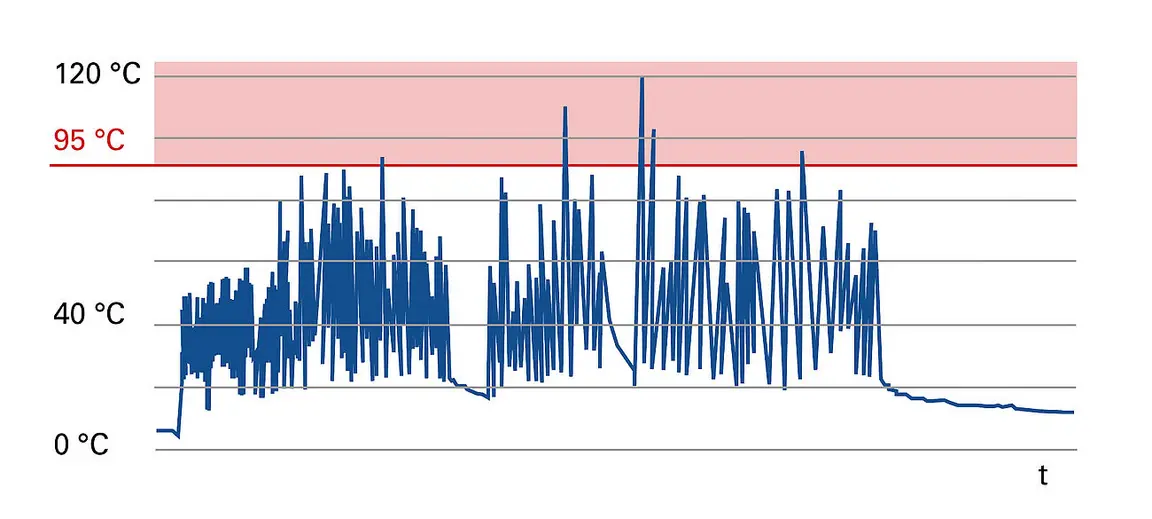
Active prevention of fires caused by damaged batteries during alternative fuel production thanks to Lindner's Fire Prevention System (FPS)
One of the most pressing recent issues in the mechanical processing and conversion of waste into solid recovered fuels (SRF) is the high fire risk. This is largely due to a constantly increasing number of lithium batteries in the general waste collection. If damaged, a chemical reaction is often initiated, which leads to incredibly high temperatures. This may cause severe damage to facilities and plants and, in the worst case, start a major fire. To minimise such fire hazards, Lindner‘s FPS (Fire Prevention System) detects overheated particles in the material stream, cools them to a safe temperature and makes sure that objects that cannot be cooled can be safely removed by hand.
Spittal an der Drau/Austria, August 2020. Whether smartphones, cars or toothbrushes – in today's digitalised, mobile society it's hard to imagine life without batteries. Billions of them are used for countless applications. According to the Austrian Chamber of Commerce’s information website ‘lithium-info.at’ (Austrian Chamber of Commerce, 2019), about 4,700 metric tons of rechargeable batteries are sold annually in the Alpine Republic, 40% of which are lithium batteries. Only about 45% of all batteries are disposed of correctly and, according to the University of Leoben, an estimated 1.4 million of them end up in the general waste collection every year (VOEB, 2019). The University also estimates that this figure will double in the medium term to 2.8 million (VOEB, 2019). Consequently, the risk of fire increases exponentially during mechanical processing, when converting waste into alternative fuels. Due to the technology used, lithium batteries, along with other highly flammable materials such as tar-soaked textile waste, have therefore become one of the most common hazards for serious fires.
PROBLEMS CAUSED BY MECHANICAL BATTERY DAMAGE
Just like any other energy storage cell, lithium-ion batteries (LIBs) consist of an anode and a cathode, separated by a Li-ion permeable membrane and a non-conductive electrolyte. Energy is released when the ions flow between the two electrodes or is stored in the anode when over-voltage is applied. Compared to other technologies, lithium-ion batteries have one of the highest energy densities thanks to the very high working voltage that can be generated between the anode and cathode. Ultimately, this is the problem when the battery is mechanically damaged and short circuits. If mechanical processing bends or severs the cell this could destroy the separator, producing a short circuit. This causes the voltage between the poles to drop to zero, releasing the stored energy as heat at different points. Even with apparently run-down, used batteries, the remaining energy is so high that temperatures of over 600 C may occur. Under certain circumstances this leads to an unstoppable chain reaction: the thermal runaway. The temperature spikes cause neighbouring cells in the battery to overheat and within milliseconds, to release their stored energy. This results in a fire or explosion that is almost impossible to extinguish. In this context it’s particularly problematic that the thermal runaway is delayed and cannot take place immediately after the mechanical damage. In SRF production this means a higher risk of fire throughout processing. The worst-case scenario is for the damaged battery to end up in the fuel storage bunker, where it could cause a devastating fire. Even if the battery burns by itself and doesn’t cause an explosion, the resulting temperatures are an enormous problem due to the fuel’s ignition point of 319 – 460 C (Lorber, 2010).